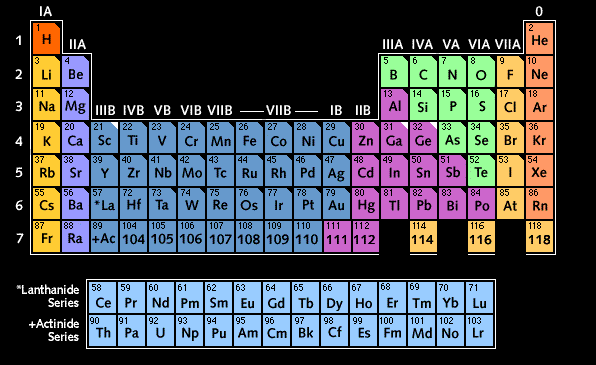
(You may have to move the frame border to see the entire table.)
Orbitals are filled with electrons from left to right. Column "0" on the right represents the noble gases, in which all orbitals on their outermost shells are filled.
Elements marked by a black triangular corner were known to Mendeleyev when he constructed the table. Elements predicted by Mendeleyev are marked by a white triangular corner.
Known elements above atomic number 103 are shown by number only. Some of them are still under dispute, and their names are not yet universally accepted. The element with atomic number 101 has been named Mendelevium.
Image: based on the Periodic Table published by the Los Alamos Laboratory, USA; public domain (US government)
The table below is from the first English edition of Dmitrii Mendeleev's Principles of Chemistry, published in 1891 and translated from the Russian fifth edition:
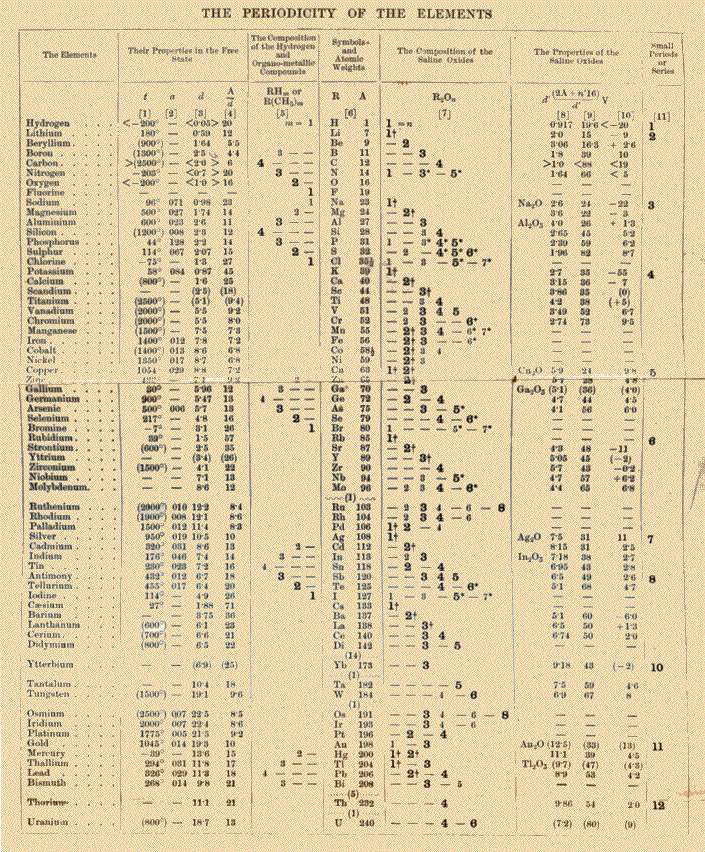
Image: public domain (Wikipedia)