An element is a substance made up of atoms with the same number of protons in its nucleus. Some elements have atoms with (by definition) the same number of protons but varying numbers of neutrons. Atoms of an element with different neutron numbers are called isotopes of that element. If such elements combine with others in a compound the molecules of the compound contain different isotopes and can be distinguished on that basis.

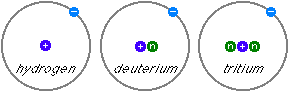
As an example, the hydrogen atom usually has one proton and one electron, which gives it the atomic number 1 and the atomic weight of approximately 1. Deuterium (D or 2H), an isotope of hydrogen, consists of atoms with one proton, one neutron and one electron. It also has the atomic number 1 (which means that it is the same element) but the atomic weight of approximately 2. Tritium (T or 3H) is the hydrogen isotope with one proton, two neutrons and one electron. It also has the atomic number 1 but the atomic weight of approximately 3. Tritium is not a stable isotope but radioactive with a half-life of 12.32 years.
Isotopes participate in chemical reactions in much the same way as the normal form of their element. Deuterium and tritium, for example, are found in water (a compound of hydrogen and oxygen, H2O). "Heavy water" is the compound in which the ordinary hydrogen atoms are replaced by deuterium (D2O). Its name indicates its elevated molecular weight: Oxygen has an atomic weight of 16, so one oxygen atom combined with two ordinary hydrogen atoms gives H2O a molecular weight of 18, while D2O has a molecular weight of 20.
The number of possible isotopes differs from element to element and varies from one (fluorine) to ten (tin).